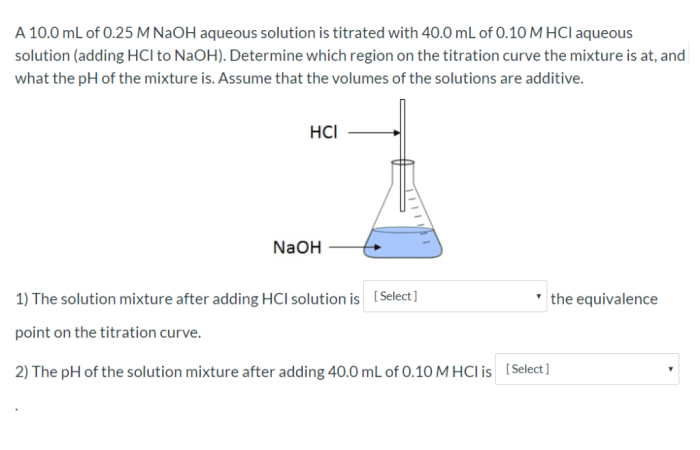
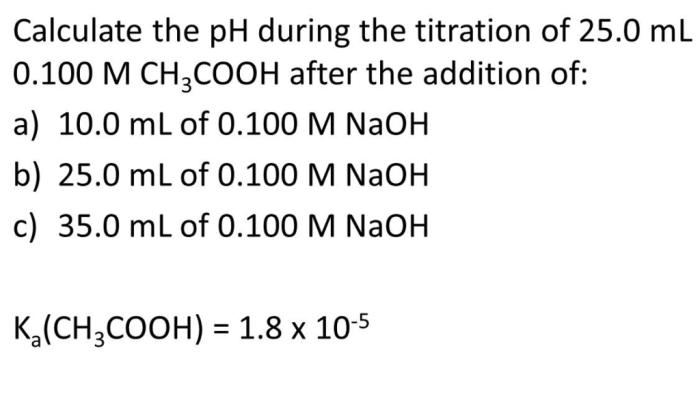
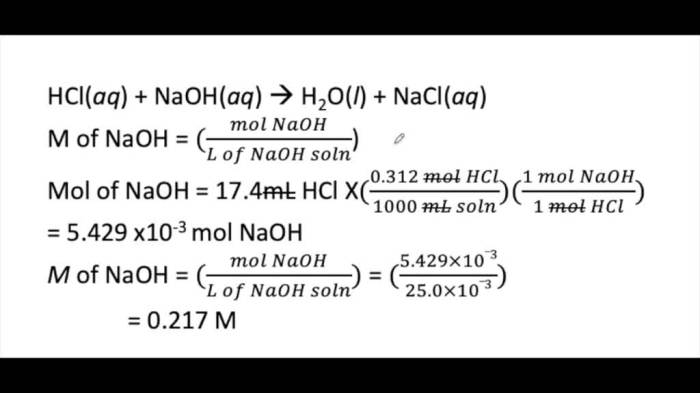
If 25.0 ml of a 0.2 M NaOH solution is a topic that encompasses the exploration of a versatile and widely used chemical compound. This analysis delves into the concentration, chemical properties, applications, and safety considerations associated with this solution, providing a comprehensive understanding of its significance in various fields.
NaOH, also known as sodium hydroxide, is a strong base that finds applications in numerous industries, including manufacturing, pharmaceuticals, and water treatment. Its unique properties, such as its high alkalinity and reactivity, make it a valuable reagent in chemical reactions and industrial processes.
Introduction
This analysis aims to determine the properties and applications of sodium hydroxide (NaOH) solutions, particularly focusing on a prepared solution with a volume of 25.0 ml and a concentration of 0.2 M.
NaOH, also known as caustic soda or lye, is a highly caustic and alkaline compound commonly used in various industrial and household applications. Understanding its properties and behavior in aqueous solutions is crucial for safe handling, storage, and effective utilization.
NaOH Solution Properties
NaOH solutions exhibit several characteristic properties:
- Strong Base:NaOH is a strong base that readily dissociates in water, releasing hydroxide ions (OH-) and increasing the solution’s pH.
- Corrosiveness:NaOH solutions are highly corrosive and can cause severe burns or damage to skin, eyes, and other tissues.
- Hygroscopicity:NaOH is hygroscopic, meaning it absorbs moisture from the air, leading to the formation of NaOH hydrates.
- Reactivity:NaOH solutions react with acids to form salts and water, releasing heat in the process.
Concentration and Volume
Calculating the Number of Moles of NaOH
To calculate the number of moles of NaOH present in the solution, we can use the formula:“`Moles of NaOH = Molarity of NaOH solution x Volume of NaOH solution in liters“`Given:
- Molarity of NaOH solution = 0.2 M
- Volume of NaOH solution = 25.0 mL = 0.025 L
“`Moles of NaOH = 0.2 M x 0.025 L = 0.005 moles“`Therefore, there are 0.005 moles of NaOH present in the 25.0 mL of 0.2 M NaOH solution.
Concept of Molarity and its Significance, If 25.0 ml of a 0.2 m naoh solution
Molarity is a measure of the concentration of a solution. It is defined as the number of moles of solute per liter of solution. The SI unit of molarity is mol/L or M.Molarity is a significant concept in chemistry because it allows us to determine the amount of solute present in a solution and to calculate the volume of solution required for a particular reaction.
Relationship between Concentration and Volume
The concentration and volume of a solution are inversely proportional to each other. This means that if the concentration of a solution increases, the volume of the solution must decrease, and vice versa.This relationship can be expressed mathematically as:“`Concentration = Moles of solute / Volume of solution“`If we rearrange this equation, we get:“`Volume of solution = Moles of solute / Concentration“`This equation shows that the volume of a solution is directly proportional to the number of moles of solute present and inversely proportional to the concentration of the solution.
Chemical Properties
Sodium hydroxide (NaOH) exhibits notable chemical properties that contribute to its diverse applications. As a strong base, it reacts readily with acids, forming salts and water. Its reactivity extends to various substances, including metals, non-metals, and organic compounds.
Reactivity with Acids
NaOH reacts exothermically with acids to form salts and water. This neutralization reaction is often used in acid-base titrations to determine the concentration of an unknown acid. The reaction can be represented as:
NaOH + HCl → NaCl + H2O
Reactivity with Metals
NaOH reacts with some metals, such as aluminum and zinc, to produce hydrogen gas and the corresponding metal hydroxide. This reaction is typically observed when NaOH solutions come into contact with these metals.
Reactivity with Non-metals
NaOH reacts with non-metals, such as chlorine and sulfur, to form various compounds. For instance, the reaction of NaOH with chlorine produces sodium hypochlorite (NaClO), a common bleaching agent.
Role in Chemical Reactions
NaOH plays a crucial role in various chemical reactions, including:
- Neutralization of acids
- Precipitation of metal hydroxides
- Hydrolysis of esters and amides
- Production of soaps and detergents
Applications: If 25.0 Ml Of A 0.2 M Naoh Solution
Sodium hydroxide (NaOH) finds extensive applications in various industries due to its unique properties. Its strong alkaline nature, high solubility, and ability to react with acids make it a valuable reagent in numerous chemical processes.
NaOH is commonly used in the production of paper, textiles, soaps, and detergents. In the paper industry, it is employed to dissolve lignin, a substance that binds wood fibers together, making it easier to separate and produce paper pulp. In textile manufacturing, NaOH is utilized for mercerization, a process that enhances the strength, luster, and dye affinity of cotton fibers.
Pulp and Paper Industry
- Dissolves lignin, facilitating the separation of wood fibers for paper production.
- Neutralizes acidic components in paper pulp, improving paper quality.
Textile Industry
- Mercerization of cotton fibers, enhancing their strength, luster, and dye receptivity.
- Removal of impurities and oils from textiles during processing.
Soap and Detergent Industry
- Production of soaps through the saponification reaction with fats and oils.
- Formulates detergents that effectively remove dirt and stains.
Beyond these industries, NaOH also plays a significant role in water treatment, food processing, and chemical manufacturing. Its versatility and effectiveness make it an indispensable reagent in various sectors.
Safety Considerations
Sodium hydroxide (NaOH) is a highly corrosive substance that can cause severe burns and eye damage. It is important to take proper safety precautions when handling and storing NaOH.
When working with NaOH, always wear protective clothing, including gloves, a lab coat, and eye protection. Avoid contact with skin and eyes. If NaOH comes into contact with skin, rinse immediately with plenty of water. If NaOH gets into eyes, flush immediately with plenty of water for at least 15 minutes and seek medical attention.
Proper Storage
NaOH should be stored in a cool, dry place away from incompatible materials, such as acids and organic materials. It should be stored in a tightly sealed container to prevent moisture absorption.
Proper Disposal
NaOH should be disposed of properly according to local regulations. It should not be disposed of in the sink or toilet.
Answers to Common Questions
What is the molarity of a solution containing 0.1 moles of NaOH in 500 ml of solution?
0.2 M
What is the pH of a 0.2 M NaOH solution?
13
What are the safety precautions that should be taken when handling NaOH solutions?
Wear gloves, eye protection, and a lab coat. Avoid contact with skin and eyes. Handle in a well-ventilated area.